LAB AUTOMATE TECHNOLOGIES MAKES THE FOLLOWING ACCESSORIES FOR THE ACI AND THE NGI:
- Silicone Mouth Piece adapters and O-rings
- One micron Stainless Steel Wire Mesh Filter Stage for ACI
- One micron Stainless Steel Filter Mesh for NGI
- USP Induction Port
- NGI Induction Port
- DUSA Tubes
We custom design and manufacture accessories at a reasonable cost—please get in touch with us.
MOUTH PIECE ADAPTERS:





Our Silicone mouthpiece adapters have been assayed for interference with (1) HPLC assay of fluticasone and salmeterol xinafoate pMDI, the diluent being water, methanol and acetonitrile in the ratio of 30:35:35 and (2) HPLC assay of albuterol sulphate pMDI, the diluent being water and methanol in the ratio of 50:50 v/v. The assay outputs show no interference as can be seen below:
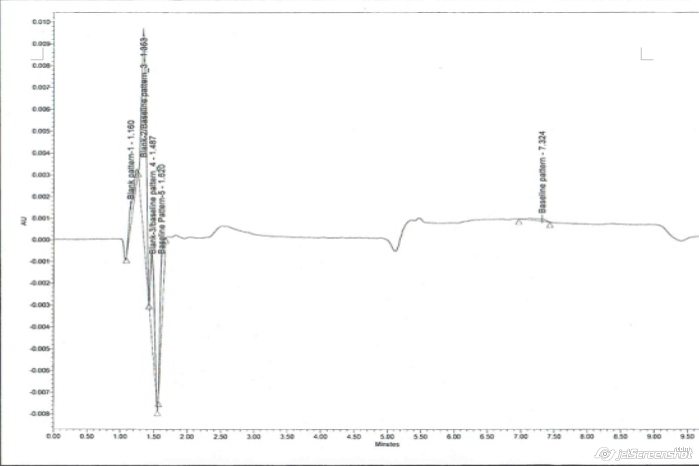
Fig 1
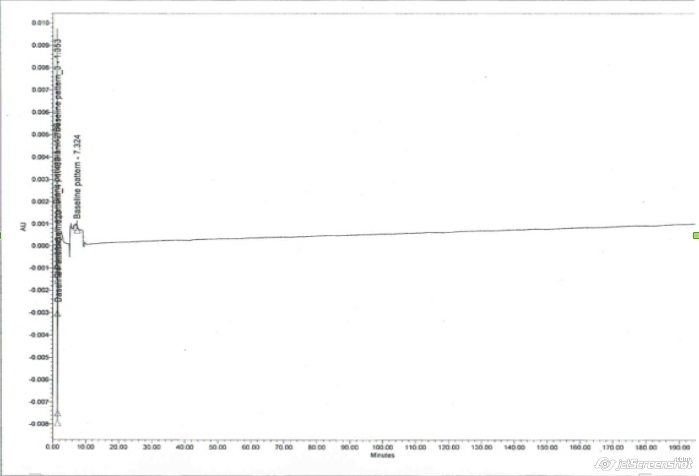
Fig 2
Interference study of mouthpiece adapter for fluticasone (Fig 1) and albuterol sulphate (Fig 2)
Mouth piece adapter is typically made of silicone and interfaces the Inhaler to the Induction Port.
It is required that:
- No chemical leach from the Mouthpiece into adapter when drug is dosed from the Inhaler into the ACI or the NGI
- No air leakage at the junctions of the Mouth Piece Adapter
- Mouthpiece adapter be easy to mount
Top of the line mouthpiece adapters sold by competitors start leaching impurities after four to five months and have to be replaced.
Lab Automate’s mouthpiece adapter was extensively tested by Sun Pharmaceuticals over a two year period and showed superior fit and performance. No impurities were detected as seen in HPLC data.
When the price of the mouthpiece adapter is evaluated, the following must be considered:
- Replacement cost
- Cost of failed tests ($500-900 per test) due to leaching. If ACI/NGI test shows impurities from mouthpiece adapter, the test data has to be discarded.
Making of Mouth Piece Adapters:
Making mouthpiece adapters is a very technical and involved job. Both the material of construction and the fit are very important. The material of construction must not leach out and show interference in the HPLC assay. If the fit is improper, there is air leakage so that 100% of the air does not come through the Inhaler into the cascade impactor. This particularly impacts the particles in the fine particle fraction range and increases data variability which negatively impacts the inhaler development program, for data variability in the fine particle range reduces the correlation of the impactor data with in-vitro patient data.
We take your 3D inhaler models and model the mouthpiece adapters in 3D before molding to get a very good fit the first time, with almost zero shrinkage Silicone. We strive to establish a partnership with our customers and work together so as to get a perfect product. This results in a mouthpiece adapter with leakage free fit, yet not so tight that it is difficult to mount, and which shows no interference with the product.
The cost varies, depending on the complexity and quantity required. Typically our customers pay a onetime charge for the die (316 Stainless Steel) and for each mouthpiece adapter.
We have dies for making mouthpiece adapter that fits into Cipla's Beclate and Flohale inhalers, Suns' Starhaler and Flovent.
STAINLESS STEEL FILTER FOR THE ACI – Cost Reduction Opportunity
Andersen Cascade Impactors are used to conduct a FDA required test called the APSD test. APSD stands for Aerodynamic Particle Size Distribution Determination. Between two to four APSD tests are carried out per ACI per day. A disposable 81mm diameter glass fiber filter has to be used for each test. We can reduce this reoccurring expense to zero in a matter of weeks.
Lab Automate has developed a 316 stainless steel woven wire mesh filter of 50 microns thickness to replace the single use filter in the Andersen Cascade Impactor. It is projected to last the lifetime of the Andersen Cascade Impactor, when used with normal laboratory level of care. It pays for itself in a few weeks, and thereafter the filter cost is zero.
This filter is embedded in the Filter Stage of the ACI. The whole unit is sold as a drop in assembly. The Filter Stage is made of 316 stainless steel. It can also be made of Titanium or Aluminum, if required. This Filter Stage has been tested by several pharma companies and they have not seen interference in their assays.
We check the filter material for purity, and interference with the assay. Our ACI filter stage is an exact replica of a traditional ACI filter stage with the filter built in. There is no bypass airflow around the filter o-ring, as happens in case of the replaceable filter. We provide a mensuration certificate for our filter stage



STAINLESS STEEL FILTER FOR THE NGI – Cost Reduction Opportunity
We can replace the NGI MOC with either 0.5 microns or 1 micron mesh Stainless Steel Filter. We have been engaged by a leading European company to do so. This removes the need for an external NGI filter and improves the reported Mass Balance.


INDUCTION PORTS:

USP INDUCTION PORT
The USP Induction Port is used with the ACI (Andersen Cascade Impactor). It is a L shaped pipe as seen on the picture on left. At one end it is connected to the ACI and at the other end a Mouth Piece Adapter is connected to it. The Inhaler is connected to the Mouth Piece Adapter. We model it in 3D and use numerically controlled machines to make it out of the best quality 316 stainless steel. We achieve metal to metal seal at the junction of the “L” and do not use any sealing compound. There is no possibility of a leak or foreign material leaching into the drug.

NGI INDUCTION PORT
The NGI Induction Port is used only with the NGI (Next Generation Impactor), and is made of 316 stainless steel. The golden end is a coating of Titanium Nitride and is plugged into the Next Generation Impactor. The two arms are welded using electron beam welding, where depth of weld penetration is very tightly controlled. The two ends are sealed after welding and the unit is tested for leaks. The Mouth Piece Adapter is plugged into the other end.
DUSA Tubes

The DUSA tube shown above is made of pure non-porous Delrin. We make DUSA tubes to order out of customer specified material. Optional 316 stainless steel woven wire mesh filter which cost breaks-even in 10-12 weeks. Thereupon cost of filter is zero
