Our History
Lab Automate Technologies Inc was founded March 2006, and our Vision is to improve the reliability and speed of pharmaceutical laboratory and manufacturing processes, so that efficacious remedies can be brought to market sooner and at lesser cost. We are headquartered in US and have an operating division in India.
We are experts in developing Test Automation Systems for Inhalation Drug Products, Precision Powder & Liquid Filling Technologies, and Material Handling Technologies—for lab scale as well as commercial scale.
With a library of technologies consisting of mechanical, electronic, imaging, and software modules, we can produce custom products, faster and better than most anyone. In terms of areas of expertise, they are :
Inhalation Products Testing Automation
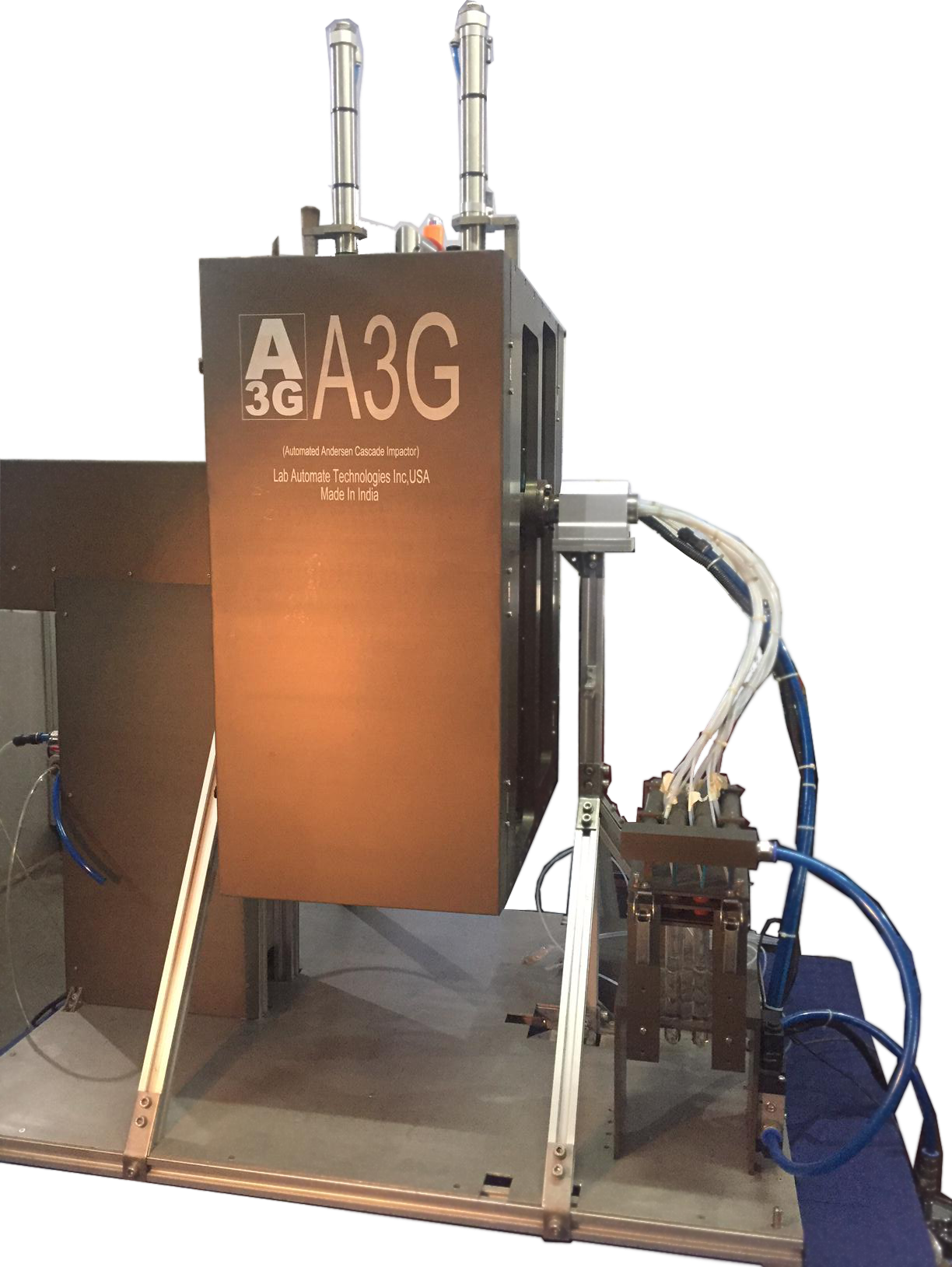
- Andersen Cascade Impaction Automation Our A3G Technology
- Emitted Dose Testing Automation
- Detection of Foreign Particulate Matter in emitted Dose from Inhalers
- Karl Fischer testing automation
- Dose Wasting Automation for dry powder & aerosol based metered dose inhalers
Precise and Automated Dispensing of a very wide range of powders in a variety of containers. These systems find use in High Speed Precision Powder Filling & Assembly Lines, Pharmaceutical development processes such as Enzymatic Screening, Selection of Excipients, dispensing of powders for Solubility Screening, & Salt Selection etc.
Our Mission
Our Team

Rajesh K. Maheshwari CEO and founder of Lab Automate Technologies, has successfully developed award winning automation technologies and systems and across functional areas in the pharmaceutical industry from early stage Discovery to Manufacturing (including a semi-automated, modified, ACI) over the past 26 years. He has successfully automated hard-to-automate processes in the areas of drug inhalation testing and drug delivery systems (including a non-CFA/HFA inhaler).As a Director Equipment Technology at MannKind Corporation he led the development of a new class of low weight powder filling technologies–a GMP compliant electro-mechanical powder dispenser, which can dispense from 2mg to 15 mg powder and weigh them in one second to ±10ug. As an Engineering Manager at Schering Plough he led the development of twelve Automation Projects—the structure of Alpha-Interferon was discovered using a single crystal grown on a Robotic Protein Crystallization system he led the development of.
Rajesh has several patents and publications to his credit. Rajesh has an MBA from Rutgers, MSEE in Computer Engineering, RPI &, BSEE in Electrical Power Engineering BIT

Krishna Maheshwari Vice President of Business Development is responsible for Product & Business Development. Krishna has an MBA from Harvard Business School & Masters in Computer Engineering and BS, Computer Science from Cornell University. Krishna has held positions with varying levels of responsibilities at Millipore, Symantec, Amazon, Microsoft and at IBM. At Microsoft, he worked on developing strategy for emerging markets. He supported sales transformation and financial justification for the Asia, Pacific, and European regions for Symantec Corporation.
Our Advisory Board

Dr. Richard Dalby is a Dean’s Distinguished Educator, Professor of Pharmaceutical Sciences, as well as the Associate Dean for Academic Affairs at the University of Maryland School of Pharmacy. He is a Fellow of the American Association of Pharmaceutical Scientists.
He has published more than 50 peer reviewed papers and approximately 130 abstracts related to aerosol technology, authored several book chapters, and spoken at many national and international meetings. He is an inventor on three patents concerned with novel MDI formulations, a reviewer for many international journals and a frequently sought after industrial and FDA consultant.
Dr. Dalby is the co-organizer, publication coordinator, editor and Exhibition moderator at the Respiratory Drug Delivery (RDD) Symposium, which is the world’s premier pharmaceutical aerosol meeting attended by researchers, executives and regulatory personnel. The resulting proceeding exceeding 1000 pages are the most up-to-date reference on advances associated with medical aerosol drug delivery.
Richard’s aerosol research, which encompasses novel pulmonary and nasal formulation development, device design and product testing, is founded on his Ph.D. work on sustained release metered dose inhalers, and industrial experience as a Formulation Scientist with Fisons (now Sanofi Aventis).
He has a Ph.D. in Pharmaceutical Sciences from the University of Kentucky (1988) (supervised by Dr. Peter R. Byron).

Dr.John Hart has been involved in leading the development of respiratory drug delivery systems for 25 years. He was the COO of Meridica, which developed a hardware-based inhalation system and when Meridica was acquired by Pfizer, he joined them as a Sr. Director. Prior to that he was Sr. Director in Schering-Plough in Inhalation Drug development and led the development of Schering’s dry-powder inhaler. John has held the position of World-Wide Director of Product Development for Rhone-Poulenc Rorer, involving most dosage forms, and in various positions in Fisons Pharmaceuticals. John was the Chairman of the International Pharmaceutical Consortium for Toxicology Testing of HFA 227 from 1990 to 2001.

Dr.Pravin Soni is a senior consultant and founder of PharmaCRO, LLC (www.pharmcro.com), with broad experience in development, manufacture and registration of novel drug, device, and combination medical products, emphasizing value creation through intellectual property development.
As VP Product R&D at Alexza Pharmaceuticals he led the development of a novel inhalable drug delivery system, the Staccato inhaler, for systemic drug delivery via the deep lung. Five different products based on Staccato technology were developed for the treatment of migraine, panic attack, breakthrough pain, insomnia and agitation under Dr. Soni’s direction, and currently these products are in various stages of clinical evaluation. Prior to Alexza, Dr. Soni was at Cygnus Inc where he led the development of the first commercial non-invasive, automatic and continuous blood glucose monitoring system, the GlucoWatch Biographer. At Cygnus he also led the development of a 7-day contraceptive transdermal system that was marketed by Johnson & Johnson as Evra.
Dr. Soni is co-inventor on fifty seven (57) US and foreign patents, and has co-authored nineteen (19) publications/poster presentations. He holds a Ph.D .in Macromolecular Science and Engineering from Case Western Reserve University.

Dr. Rod Woods has 30 years of analytical, quality and process experience in the Chemical and Pharmaceutical industries. Currently Director of Process Analytical Technology at Mannkind Corporation. Dr. Woods has in the past been senior management of Quality Control , Analytical Development, Quality Assurance and Chemical Technology for Mannkind. Prior to Mankind had management positions in Cambrex and Cyanamid . Dr. Woods has a Ph.D. in Analytical Chemistry from Seton Hall University.